Abstract
Plasma cell dyscrasias (PCDs), including multiple myeloma (MM), light-chain amyloidosis (AL), and POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes), are malignancies of plasma cells but with diverse clinical features. Although they have the same precursor stage of monoclonal gammopathy of undetermined significance (MGUS), their shared mutational pathogeneses remain understudied.
To investigate the common mutational signatures with clinical significance, we subjected bone marrow plasma cells from patients with MM (n=163), AL (n=121), POEMS syndrome (n=67), and MGUS (n=13) using a targeted gene sequencing (TGS) panel of 370 genes. This TGS panel is designed on the basis of our previous research on whole-exome sequencing of AL (Huang XF et al, Amyloid, 2020) and POEMS syndrome (Chen J et al, Leukemia, 2021).
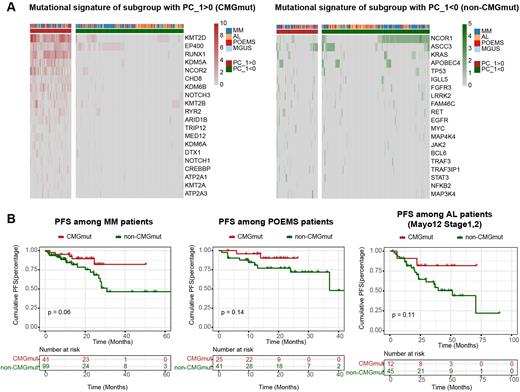
Strikingly, the principal component analysis of the top 100 variably mutated genes revealed two major subgroups among patients with PCDs: one subgroup with a high mutational burden of chromatin-modifying genes (namely the CMGmut subgroup), and the other subgroup with a subtly higher burden of classical mutations in MM (namely the non-CMGmut subgroup) (Figure 1A). The most heavily mutated chromatin-modifying genes in the CMGmut subgroup of PCDs included KMT2D, EP400, RUNX1, KDM5A, etc. The Least absolute shrinkage and selection operator (LASSO) regularization analysis revealed that the high mutational burden of RUNX1 and KMT2D was the biomarker to distinguish the CMGmut subgroup from the non-CMGmut subgroup.
As for clinical significance, the patients with MM, early-stage AL (Mayo12, Stage 1,2), or POEMS syndrome of the CMGmut subgroup were more likely to have prolonged progression-free survival than those of the non-CMGmut subgroup (Figure 1B). Also, the patients with MM or AL of the CMGmut subgroup showed inferior responses to the first-line CyBorD (cyclophosphamide, bortezomib, and dexamethasone), whereas the patients with MM of the CMGmut subgroup showed non-inferior responses to the first-line VRD (bortezomib, lenalidomide, and dexamethasone).
In summary, TGS panel-based mutational profiling provided mechanistic insights into a subgroup of patients with PCDs characterized by a high mutational burden of chromatin-modifying genes and relatively indolent clinical features. Our findings enable the identification of these patients who will potentially benefit from epigenetic therapy in future clinical management.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal